
Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

LB0005 MULTICENTRE, RANDOMISED, OPEN-LABEL, ASSESSOR-BLINDED, PARALLEL-GROUP HEAD-TO-HEAD COMPARISON OF THE EFFICACY AND SAFETY OF IXEKIZUMAB VERSUS ADALIMUMAB IN PATIENTS WITH PSORIATIC ARTHRITIS NAIVE TO BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC ...

A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis - ScienceDirect
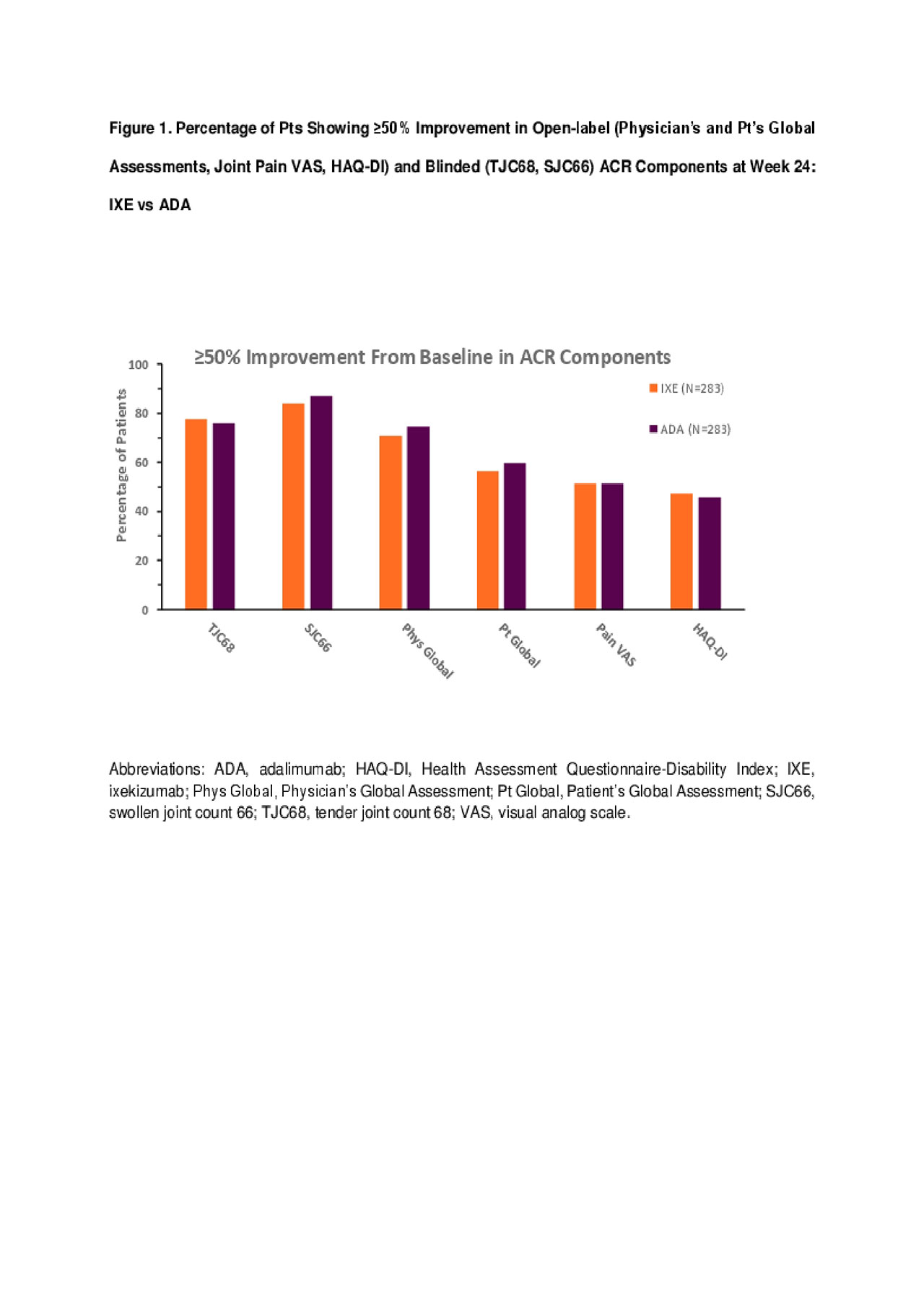
Ixekizumab Demonstrates Improvement Comparable to Adalimumab Across ACR Components in Biologic-Naïve Patients with Psoriatic Arthritis - ACR Meeting Abstracts

Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial - The Lancet

Fillable Online accessdata fda LABEL. TALTZ (ixekizumab) injection, Eli Lilly and Company - accessdata fda Fax Email Print - pdfFiller

Long‐term efficacy and safety results from an open‐label phase III study (UNCOVER‐J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab - Umezawa - 2019 - Journal of

Long‐term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: subgroup analyses of an open‐label, phase 3 study (UNCOVER‐J) - Okubo - 2019 - Journal of the

Figure 3. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology
Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials | SpringerLink

Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52 | Annals

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect
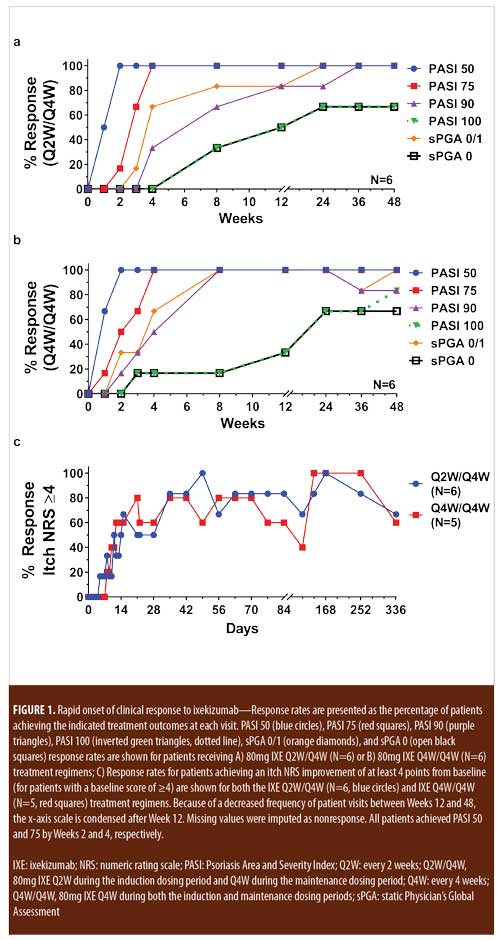
Early Onset of Clinical Improvement with Ixekizumab in a Randomized, Open- label Study of Patients with Moderate-to-severe Plaque Psoriasis – JCAD | The Journal of Clinical and Aesthetic Dermatology

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)









