ACTAVIS: Teva Announces Updated Indication and Vial Presentation for GRANIX® (tbo-filgrastim) Injection in United States | American Pharmacy News

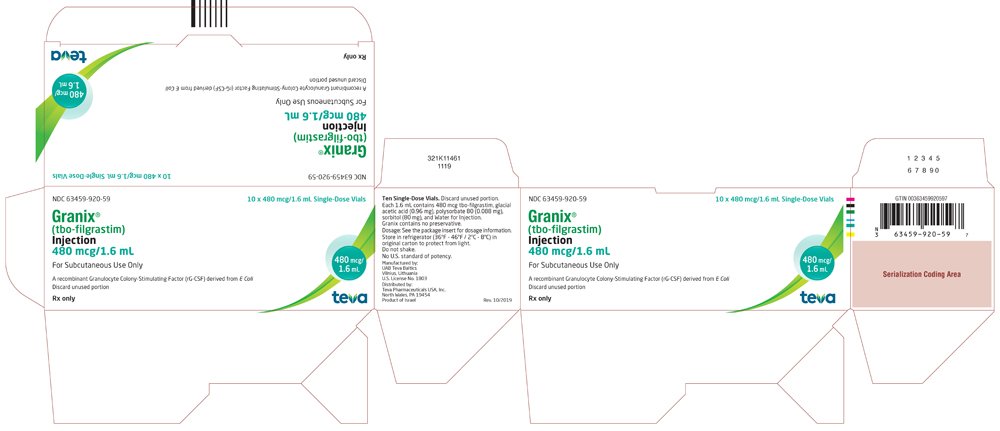
TEVA PHARMACEUTICALS USA, INC: Teva Announces Updated Indication and Vial Presentation for GRANIX® (tbo-filgrastim) Injection in United States | American Pharmacy News

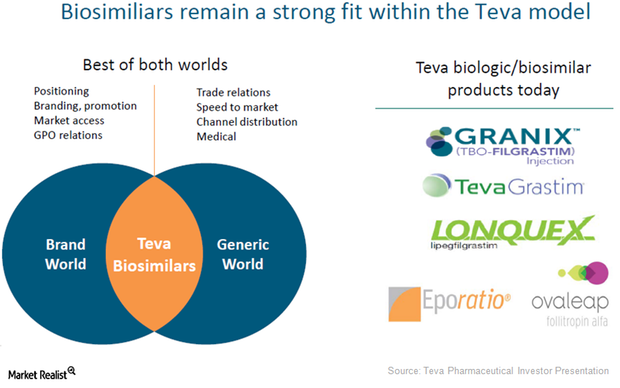
Biosimilar… but different: FDA tweaking nonproprietary name guidance - BioProcess InternationalBioProcess International
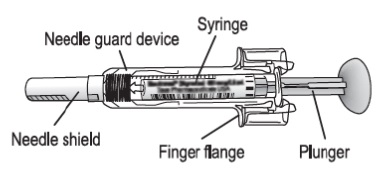
GRANIX (TBO-FILGRASTIM) INJECTION Trademark of TEVA Pharmaceutical Industries Limited Serial Number: 86040042 :: Trademarkia Trademarks